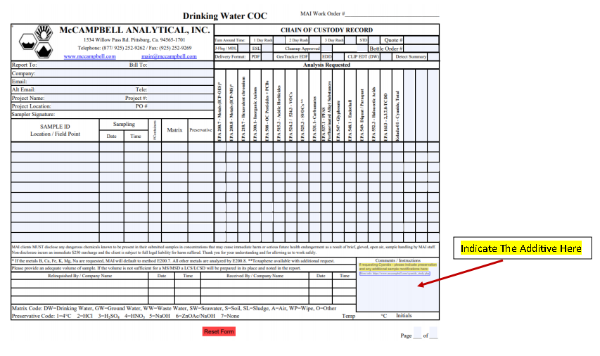
Field Preservation
Field preservation options are of more significance to CN-
measurement in waste water (WW) rather than drinking water (DW) because of higher total organics, more complex
WW sample matrix and lower required CN- reporting limits in CA (5ppb WW vs 100ppb CN- in DW).
Field Treatment
Field treatment of samples immediately after collection is in part mandatory (basic pH preservation,
strip paper testing for oxidizers & sulfide and field treatment if present) and in part optional (strip paper
testing, sulfamic acid treatment for N0 2 /NO 3 -, ethylenediamine (EDA) treatment for carbonyls, Ca(OH) 2
treatment for high carbonate). MAI repeats the oxidizer & sulfide tests before lab analysis as well as
verifying pH compliance.
We recommend the following sequential treatments as a general procedure. For the following
discussion the oxidant is assumed to be chlorine/chloramines. Unusual samples or alternate facility
procedures may require different treatments. Omit the dechlorination step if oxidizer has not been
added at the facility.
Instructions
1. Perform Lead acetate (PbAc) strip paper determination of sulfide on a native sample aliquot. Add 1 drop of sample to freshly moistened (acetate buffer) strip paper and record the reading. If sulfides are positive, then dilute the sample with reagent water until no longer positive. Include reagent water blank with sample set and clearly note dilution factor on the COC. MAI can provide reagent water upon request.
2. Perform chlorine strip paper oxidizer determination on a native sample aliquot. Add 1 drop of sample to freshly moistened (acetate buffer) strip paper and record the reading. If overrange on the 1) 0-10ppm strips use the qualitative KI strips. The more accurate & sensitive DPD measurement of chlorine is used by some facilities.
3. Add sufficient Ascorbic Acid to destroy chlorine/chloramines/monochlorosulfamate. The initial amount of chlorine present was determined by the oxidizer or DPD test, and we recommend adding at least 1.5x that amount of Ascorbic Acid; Sodium Thiosulfate is not recommended b/c sample pH<2 and Sodium Thiosulfate is known to form S(0) at acid pH. The stoichiometric weight of the dechlorination agent needs to be added in excess to ensure complete dechlorination. Future data may support the addition of greater excess, for example, 2-30x, to allow a wider margin of error and possibly avoid the need for precise chlorine measurement. The stoichiometric 1:1 amount needed, in mg dechlorination agent per mg Cl2, 1.5x the stoichiometric amount in a given volume of water, and methodological amounts are tabulated next. Numbers are rounded.
| mg/L of AA per mg/L of Cl 2 for Stoichiometric Reduction. All reactions are 2 electron/mole transfers |
| Chlorine source; Cl 2 , MW= 70.9 |
Stoichiometric AA,mg per Cl 2 ,mg; AA, MW= 176.1 |
DW (<5mg Cl 2 /L) per 250ml; 1.5x Stoichiometric AA,mg per Cl 2 max,mg |
WW (<50mg Cl 2 /L) per 250ml; 1.5x Stoichiometric AA,mg per Cl 2 max,mg |
Method prescribed AA,mg/L |
| |
2.3 mg AA/ mgCl2 |
4.5mg/8oz amber |
45mg/8oz amber |
25-150mg/8oz amber |
| mg/L of Sodium Thiosulfate per mg/L of Cl2 for Stoichiometric Reduction. All reactions are 2 electron/mole transfers (Sodium Thiosulfate use is not recommended) |
| Chlorine source; Cl2, MW= 70.9 |
Stoichiometric TS,mg per Cl2,mg; AA, MW= 158.1 |
DW (<5mg Cl2/L) per 250ml; 1.5x Stoichiometric TS,mg per Cl2max,mg | WW (<50mg Cl2/L) per 250ml; 1.5x Stoichiometric TS,mg per Cl2max,mg/th>
| 500 & 600 series method prescribed TS,mg/L |
| |
2.0 mg TS/ mgCl2 |
4.0mg/8oz amber |
40mg/8oz amber |
80mg/8oz amber |
If chlorine is not measured, then add 0.6g AA/L sample (OIA 1677-09; 0.1-0.6g ASTM D7365 s8.3.9.1) ≈ 150mg/8oz amber. From the table above this is 3x molar excess of the largest amount of Cl2 used in WW and 30x that used in DW. MAI can provide 2@ 8oz ambers each containing 150mg AA if desired. Cap & gently invert 3x. Now the sample is dechlorinated; NO2- is absent.
4. Wearing gloves, pour the sample into the treatment container with sulfamic acid (solid) & fill to the top (pH is <2). Observe if sample bubbles and if so discard a few mLs to prevent excess gas pressure. Cap, and gently invert 2x to mix. This removes NO2- by reducing it to N2. It likely drives all free chlorine & chloramines to monochlorosulfamate.
5. Carefully pour the above treated sample into a new bottle provided by MAI that contains
enough NaOH to raise sample pH to 10.5 for WW and 12.5 for DW. Leave minimal headspace
(few mls or 5v% if freezing). Cap & store on ice. EDA is not recommended as it was found to
create significant CN- (up to xxx ppb) under these conditions.
Samples must be marked for 24 TAT if AA was used as the dechlorination agent.
6. Record all preservations and sample modifications on the COC
Samples must be marked for 24 TAT if AA was used as the dechlorination agent.
MAI can provide customized reagents, test papers, and bottles for any facility-designed treatment procedure.
A field blank is recommended with each sample set. MAI agrees with Standard Methods in recommending an MS for EACH SAMPLE, initially and when site preservative procedures are changed, and for problematic samples. After site behavior for CN- has been established the recommended spiking frequency is reduced to one MS & MSD for each sample set. We can provide instructions for spiking as well as an MS kit if needed.
If you would like to receive a standard or customized sample kit from us or would like to discuss this information letter, please contact us.
Appenidx 1: Standard MAI Field Preservation Kit per COC
PbAc test strip papers for sulfide screening.
Chlorine & KI oxidizer strip papers (0-10 & 0-50mg/L range)
- ~10ml acetate buffer used to moisten strip papers prior to measurement for sulfide & oxidants
[*Acetate buffer solution, pH 4.0: Dissolve 146 g anhydrous NaC2H3O2, or 243 g NaC2H3O2 3H2O, in
400 mL distilled water, add 480 g conc acetic acid, and dilute to 1L SM2023 4500-Cl C3e] +
disposable pasteur pipettes + bulb.
1@ 500ml clear plastic sampling container per sample
2@ 8 oz (236.6ml) amber plastic per sample each containing 1g reagent grade sulfamic acid.
2@ 8oz amber per sample each preloaded with 150mg AA (=600mg AA/L) or amount specified by client
- OR ~10g granular reagent grade AA for client addition at facility.
2@ 8 oz amber plastic per sample containing sufficient NaOH to bring sample pH~10.5 (WW) or ~12.5
- (DW). Fill to top minus ~3-5ml for thermal expansion. This is the sample container that is shipped to
the lab.
MS kit if requested. 2.37mg CN- /L (from NaCN in DI) @ pH=12 spiking solution with 1 ml syringe. Add
- 1ml to full 8oz amber plastic to achieve 10ppb CN- spike. Enough volume of spiking solution is
provided to make requested spikes. Add to container immediately after dechlorination treatment.